Overview:
R & D application to SIR contains the following elements:
- Applicants take part in SIR's routine for R & D applications submitted to SIR
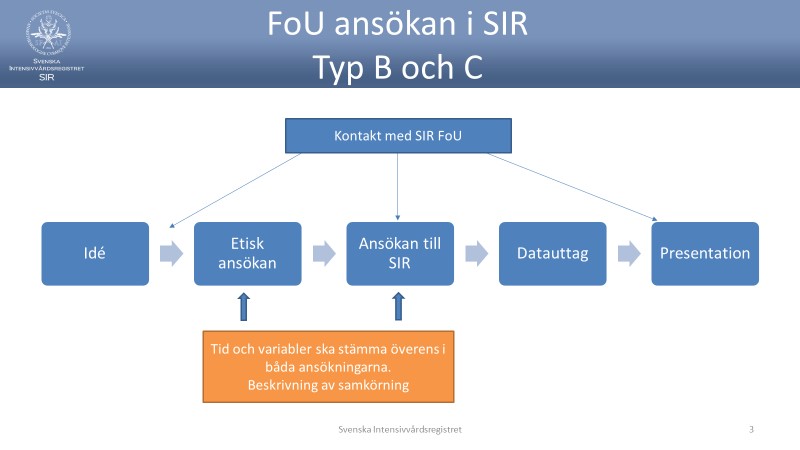
- For applications Type B and C, applicants fill in: Request for data extraction from the Swedish Intensive Care Register
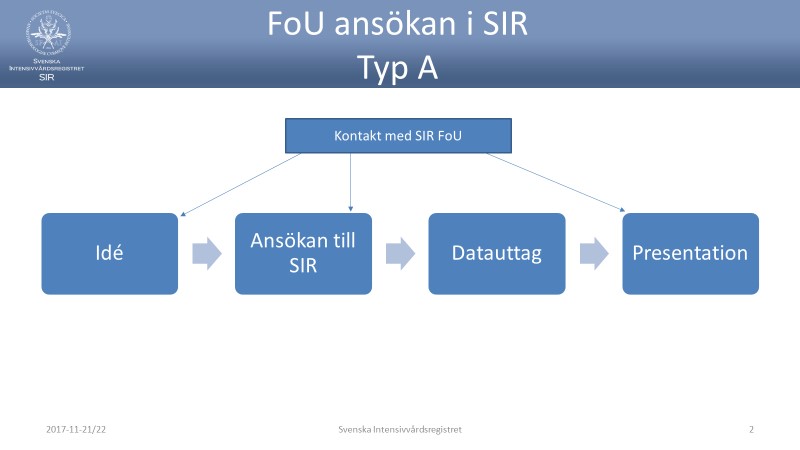
- The application is sent to SIR. All documents and forms can be sent by email. For application Type A, the application can be entered directly in an email
- The application is prepared by SIR-R & D
- The application is discussed by telephone with applicants, SIR-IT and SIR-R & D to assess how data extraction can be done in the best possible way, and what the list of variables should contain. Information is provided on SIR's publication guidelines, annual follow-up, ClinicalTrials.gov and STROBE
- The application is assessed by SIR according to different procedures for applications of types A, B, or C
- A decision on data extraction is taken by SIR. Type A may be decided by the Chairman or CEO. Types B and C are decided by the board
- The decision is documented, upon application of Type B or C, a contract is signed with the applicant of the Chairman of the Board or the CEO
- SIR-IT provides data collection after the contract is written. The SIR working time for this are invoiced to the applicant
- SIR-IT delivers data to the applicant or to the The National Board of Health and Welfare at the time of intercourse
- SIR-R & D has annual contact with the applicant and follows up on presentations and publications
- Presentations and publications are published on the SIR website
Applications:
The following applications are prepared by SIR:
- Single application, Type A:
The typical case for a simple application may be that a department of SIR wants access to own aggregate data regarding any action or care times in a way that is not clearly available through the data output portal. Data is collected by the own department (the principal) who is also responsible for the data received. An approved research ethics application is not required for a simple Type A application. - Extended application, Type B. This generally applies to more extensive data extractions (usuallymore than one department and another principal than the clinic that is a member of SIR) including calculations or other data transformations and / or interactions, e.g. with other quality registers. An approved research ethics application is required in a Type B application.
- Application including social security number, Type C.
With such an application, applications in category B are equated, showing that data are linked to identifiable persons by means of interlinking by other means than social security numbers from SIR.
An approved research ethics application is required for a Type C application. In these cases, the trial of SIR is based on the ethical application where it must clearly be stated that the social security number is included and that the Ethical Review Board has approved this. In the application to SIR itself, the process of data management must be clearly described and, if possible, Register Centers at the National Board of Health and Welfare shall implement this and then divulge the applicant data in an unidentified state. In cases where this is not possible for various reasons, a special examination is performed on how storage, access and use are planned. If the application is granted, these areas must be detailed contractually. Upon transmission of data, responsibility for further processing of data, according to General Data Protection Regulation, data to the recipient is transferred. Recipients should then be an academic or healthcare representative with a position to guarantee resources for safe data management. In case of doubt, special inquiries are made in the case before decisions and contract writing. - Application for extended data exits on existing application.
Following the preparation of the SIR-R & D and SIR-IT case, the Board decides on increased data extraction. A new contract is not required. Once the Board has announced its decision, a renewed data extraction can be made by SIR-IT. - Application for extended data withdrawal on existing application with new ethical permission.
Following the preparation of the SIR-R & D and SIR-IT case, the Board decides on increased data extraction. A new contract is not required. Once the Board has announced its decision, a renewed data extraction can be made by SIR-IT. - Application for data from authorities.
The request is prepared by the Chairman of the Board, CEO and SIR-R & D. Following a decision of the Board, the Chairman or CEO has contact with the Authority before SIR-IT delivers data. The CEO or Chairman of the Board informs the Authority of the SIR Publication Policy.
Data collection is dated and documented in the same way as a R & D data extraction.
Single application Type A:
|
Decision by: |
Application: |
Data delivery: |
|
Chairman of the Board |
Application by email. |
To Applicant following the decision of the board and contract writing |
Extended application, Type B
|
Decision by: |
Application: |
Data delivery: |
|
Board of Directors |
Request for data withdrawal from the Swedish Intensive Care Register |
To Applicant |
|
|
An approved research ethics application is required |
Following the decision of the board and contract writing |
Extended application, Type C:
|
Decision by: |
Application: |
Leverans av data: |
||
|
Board of Directors |
Request for data withdrawal from the Swedish Intensive Care Register |
To Applicant |
||
|
|
|
Following the decision of the board and contract writing |
||
|
|
|
To The National Board of Health and Welfare or someone else who cares for intercourse |
||